Coming Soon
Designed for Modern Healthcare Needs
These innovative bags are ideal for:
- Critical care and ICU settings
- Oncology and specialty infusions
- Neonatal and pediatric patients
- Institutions aiming to reduce environmental footprint
Coming Soon
At Silica Healthcare, innovation never stops. In our continuous journey to elevate patient care and safety, we are excited to announce the upcoming launch of Non-PVC (Polyvinyl Chloride-free) Parenteral Bags — a next-generation packaging solution designed to meet the evolving needs of hospitals, critical care units and global healthcare standards.
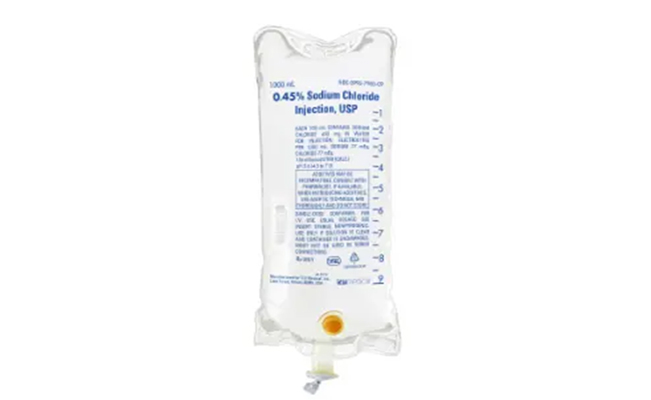
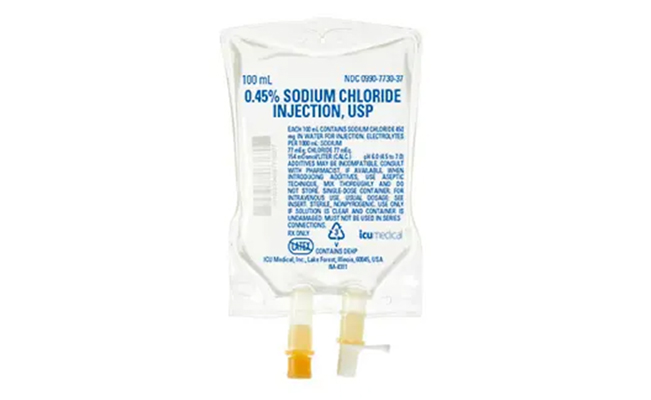
Traditional PVC (Polyvinyl Chloride) infusion bags may contain plasticizers like DEHP (Diethylhexyl Phthalate), which have raised safety concerns due to their potential to leach into solutions, especially during long infusions or high-temperature conditions.
Our Non-PVC Parenteral Bags are:
Our Non-PVC Bags are being developed using the latest in multi-layered polymer technology, ensuring:
They will be manufactured on automated BFS/FFS Sterile lines under Class 100 sterile conditions, assuring the same unmatched sterility and consistency Silica is known for.
These innovative bags are ideal for:

Mr. S.K. Dubey is the Chief Business Officer at Silica Healthcare Pvt. Ltd., bringing over 25 years of experience in the pharmaceutical industry.
His expertise lies in sales, marketing, business development and market expansion, with a strong track record in both domestic and international markets, particularly in South Asia and the Middle East. He has held leadership positions at Eli Lilly, Raptakos Brett, USV, Emcure and Albert David Ltd., consistently delivering strong commercial outcomes.
At Silica Healthcare, he leads efforts to expand the company’s commercial footprint, enter new geographies and strengthen product positioning in IV fluids and specialty segments. Known for his strategic acumen and deep understanding of customer behaviour, he has successfully driven brand growth and managed cross-functional teams in complex market environments.
His leadership blends market agility with long-term vision, making him instrumental in shaping Silica Healthcare’s growth as a competitive, future-focused pharmaceutical player.

ISBM stands for Injection Stretch Blow Molding, a cutting-edge plastic manufacturing technology used to produce high-quality, sterile and durable containers—especially in the pharmaceutical industry for IV fluids, injectables and infusion solutions.
ISBM is a high-precision, sterile and scalable technology that produces rigid, shatterproof and high-clarity containers.








Mr. Kushal Kumar is the Accounts Manager at Silica Healthcare Pvt. Ltd., Bhagwanpur, bringing over 15 years of experience in finance and accounts.
His expertise spans statutory compliance, financial reporting, credit control and taxation, with a strong command of MIS reporting, reconciliations and general ledger management. He has previously held key roles at Arsiga Konics, ATS Edutech and Team Lease Services, where he contributed to building robust financial systems.
At Silica Healthcare, he is responsible for strengthening financial controls, ensuring accurate, audit-ready reporting and maintaining full compliance with regulatory norms—contributing directly to the company’s operational efficiency and financial integrity.


Mr. Sanjay Veer is the Production Head at Silica Healthcare Pvt. Ltd., Patna, bringing over 25 years of experiencein sterile injectable manufacturing.
He holds deep expertise in large and small volume parenterals (LVP & SVP), production planning, regulatory compliance and quality assurance. He has previously served in senior leadership roles at Kilitch Healthcare, Hindustan Antibiotics Ltd., and TruElixir Lifescience, contributing to the setup and optimization of sterile manufacturing operations.
At Silica Healthcare, he leads the production of LVPs using advanced FFS technology, ensuring strict adherence to cGMP norms and maintaining regulatory audit readiness. His technical proficiency and leadership are key to building efficient, compliant production systems that meet both domestic and global quality standards.


Mr. Krishna Das Mathur is the Manager – Quality Control (Microbiology) at Silica Healthcare Pvt. Ltd., Bhagwanpur, bringing over 19 years of experience in pharmaceutical microbiology and chemical quality control.
His expertise includes sterile and non-sterile formulations, method validation, environmental monitoring and regulatory compliance. He has held key quality leadership roles at Cipla, Swiss Garnier and Ciron Drugs, where he contributed to strengthening microbiological systems and inspection readiness.
At Silica Healthcare, he is responsible for enhancing microbiological quality systems, ensuring audit preparedness and building a compliant, inspection-ready quality control framework aligned with international regulatory standards.


Mr. Rajendra Khairnar is the Head of Operations at Silica Healthcare Pvt. Ltd., bringing over 30 years of experience in pharmaceutical manufacturing and plant management.
He has led operations at Fresenius Kabi, Otsuka Pharmaceutical, Puerto Life Sciences and Sipra Remedies, with deep expertise in parenterals, Blow-Fill-Seal (BFS) technology and end-to-end process optimization. He has successfully managed production teams of over 500 professionals, ensuring operational efficiency, regulatory compliance and strong quality systems across multiple manufacturing facilities.
His core strengths include sterile manufacturing, production planning, asset utilization and audit preparedness. He is highly experienced in liaising with regulatory bodies such as the FDA, Pollution Control Board, boiler and factory inspectors, ensuring uninterrupted and compliant operations.
At Silica Healthcare, he is instrumental in strengthening operational systems, guiding technical teams and aligning production standards with global regulatory expectations. Known for his strategic vision and hands-on leadership, he plays a key role in driving long-term manufacturing excellence and sustainable growth for the organization.


Mr. Amitabh Prasad is the Director at Silica Healthcare Pvt. Ltd., bringing over 25 years of industry experience, including more than a decade in CXO-level roles.
At Silica Healthcare, he leads scalable systems development, cross-functional strategy alignment and the delivery of sustainable, innovation-led growth. His operational leadership ensures high efficiency, strong execution and unwavering commitment to quality and compliance, reinforcing the company’s foundation as a future-ready, impact-driven pharmaceutical enterprise.
Over his career, Mr. Prasad has held senior leadership positions in Whirlpool, KOHLER, SKF, Mahindra, JK Tyre and Ceat, with responsibilities spanning business strategy, marketing, sales and business development. He has successfully driven business transformations, mergers and acquisitions and market expansion across India and South Asia.
He holds an MBA from SP Jain Institute of Management, an M.Tech from IIT Delhi and a B.Tech from BIT Mesra, a strong academic blend that underpins his strategic and operational excellence.


Mr. Abhinav Mayank is the Co-founder and Director of Silica Healthcare Pvt. Ltd., leading the company’s strategic foray into pharmaceutical manufacturing.
With over 20 years of experience in global organizations, he specializes in business building, cross-functional leadership and establishing scalable, compliant operations. At Silica Healthcare, he oversees the development of advanced manufacturing facilities for IV fluids and sterile pharmaceutical products, ensuring quality, regulatory compliance and long-term sustainability.
He has held leadership roles at IBM, Bank of America, Welspun, Dainik Bhaskar and Stream, driving operational excellence and strategic growth.
Mr. Mayank holds an MBA from Nanyang Technological University, Singapore, a Project Management Certificate from SP Jain Institute, Mumbai and is a certified Project Management Professional (PMP).


Mr. Amrendra Kumar is the Chairman and Managing Director of Silica Group, known for his visionary leadership and entrepreneurial acumen.
With over 20 years of entrepreneurial experience, he has built a reputation for scaling businesses with a focus on regulatory excellence, operational integrity and national impact. He is widely recognized for his strategic foresight, particularly in energy and infrastructure and now in healthcare sector.
Mr. Kumar began his career in the IT industry with Tata Consultancy Services (TCS) and transitioned into the energy sector in 2009, Launching his first entrepreneurial venture in IT Sector followed by Oil & Gas Sector . In 2011, he partnered with Bharat Petroleum Corporation Limited (BPCL) to support the “Bottling Assistance from Private Bottlers Project” in Bihar, playing a key role in laying the foundation for LPG bottling infrastructure in Bihar and Jharkhand.
Today, under his leadership, Silica Group is the second-largest manufacturer of LPG cylinders in India and runs the nation’s largest LPG bottling network with 18 fully operational plants. Building on this strong foundation, the group is now expanding into healthcare, focusing on IV fluids and sterile pharmaceutical manufacturing, with a long-term vision to support a self-reliant healthcare ecosystem in India.
Mr. Kumar holds a Bachelor of Technology (B.Tech) degree and brings a deep, hands-on understanding of systems and operations across multiple sectors.


Mr. Arindam Deb is the Head Quality Assurance at the Bhagwanpur facility of Silica Healthcare Pvt. Ltd., bringing over 17 years of experience in pharmaceutical quality operations.
His expertise spans cGMP, data integrity, regulatory submissions and analytical quality assurance across both formulations and APIs. He has worked with leading pharmaceutical companies such as Pfizer, Fresenius Kabi and Shilpa Medicare and has supported regulatory inspections by USFDA, MHRA, EDQM and the WHO.
At Silica Healthcare, he leads analytical quality systems, reinforces laboratory compliance and drives continuous improvement to meet global regulatory standards. His leadership ensures precision, traceability and audit readiness across all analytical functions.


Mr. Ram Kumar Sharma is the Purchase Manager at the Bhagwanpur facility of Silica Healthcare Pvt. Ltd.,bringing over 16 years of experience in pharmaceutical procurement and supply chain operations.
An MBA graduate, he has strong expertise in vendor development, inventory control and material planning, with a proven ability to streamline warehouse and PPIC functions for operational efficiency.
At Silica Healthcare, he is responsible for strengthening procurement systems, improving cost-effectiveness and ensuring uninterrupted material flow to support production continuity and long-term operational excellence. His structured, results-driven approach aligns with Silica’s evolving supply chain and growth ambitions.


Mr. Jagdish Singh Sidhu is the HR Head at the Bhagwanpur facility of Silica Healthcare Pvt. Ltd., bringing over 21 years of experience in human resources across the FMCG and pharmaceutical sectors.
His core expertise includes policy design, compliance management, talent development and building resilient, high-performing work cultures. Known for his strategic mindset and people-first approach, he has consistently driven organizational effectiveness through strong team building and workforce engagement.
At Silica Healthcare, he leads initiatives to align the workforce with business goals, optimize HR systems and strengthen leadership pipelines, playing a vital role in supporting the company’s long-term growth.


Mr. Vijay Kumar Singh is the Assistant Manager – EHS at Silica Healthcare Pvt. Ltd., Bhagwanpur, bringing over 15 years of experience in environment, health and safety (EHS) management.
His expertise includes statutory compliance, fire safety, pollution control, risk assessment, audits and safety training across large-scale industrial operations.
At Silica Healthcare, he is responsible for strengthening the EHS framework, promoting a safety-first culture and ensuring full alignment with environmental and occupational safety standards across all operations.


Mr. Veermani Pandey is the Assistant Manager – Quality Control at Silica Healthcare Pvt. Ltd., Bhagwanpur, with nearly 9 years of experience in pharmaceutical quality control.
With a strong academic foundation in Chemistry, he brings hands-on expertise in analytical testing, documentation and regulatory compliance across formulations and API environments.
At Silica Healthcare, he plays a key role in strengthening laboratory systems, ensuring data integrity and maintaining consistent product quality in line with evolving industry and regulatory standards.


Mr. Raj Kumar Ojha is the Engineering Head at Silica Healthcare Pvt. Ltd., Bhagwanpur, bringing over 17 years of experience in engineering leadership within the pharmaceutical sector.
A B.Tech graduate, he specializes in utilities management, project execution and technical operations and has successfully led infrastructure upgrades and plant expansion projects across multiple facilities.
At Silica Healthcare, he is responsible for enhancing plant reliability, minimizing downtime and driving innovationin engineering systems—ensuring that manufacturing remains scalable, efficient and fully compliant with regulatory standards.


Mr. Santosh Kumar Singh is the SCM Manager at Silica Healthcare Pvt. Ltd., Bhagwanpur, bringing 15 years of experience in production, planning, warehouse coordination and Supply Chain Management.
His expertise includes Production, Planning and Inventory Control (PPIC), layout optimization and material staging, offering deep operational insight across diverse manufacturing setups.
At Silica Healthcare, he is responsible for streamlining internal logistics, enhancing equipment utilization and ensuring uninterrupted production through effective resource planning and real-time inventory control.

Locations: Lucknow, Patna, Kolkata, Nagpur, Chennai, Hyderabad, Bangalore, Delhi
Experience: 10–15 years (Pharma/Healthcare Sales & Marketing), with 3–5 years in a leadership role
Key Responsibilities:
• Lead business operations across assigned zone, ensuring achievement of sales & profitability targets
• Manage and mentor Regional Managers, Area Managers and field force teams
• Drive institutional business, retail sales and channel expansion
• Execute marketing strategies, brand promotions and ensure field execution
• Build strong relationships with hospitals, institutions and channel partners
Locations: Patna, Muzaffarpur, Ranchi, Bhagalpur, Lucknow, Kanpur, Gorakhpur, Varanasi, Guwahati, Kolkata, Nagpur & other major Indian cities
Experience: 7–10 years (Pharma/Healthcare Sales), with minimum 2–3 years as an Area Manager
Key Responsibilities:
• Manage sales & marketing operations across assigned region/state
• Lead Area Managers & MRs to achieve monthly/annual sales targets
• Ensure execution of marketing campaigns, product launches & brand visibility initiatives
• Develop distribution channels & ensure availability in hospitals, retail & institutional business
• Monitor competitor activity and conduct market analysis
Locations: Cuttack, Kolkata, Delhi, Jaipur
Experience: 3–6 years (Institutional / Hospital / Government Sales, IV Fluids experience preferred)
Key Responsibilities:
• Manage relationships with key hospitals, government and institutional accounts
• Achieve sales targets through customized account strategies
• Negotiate tenders, contracts and pricing for institutional business
• Ensure supply chain coordination & product availability in assigned accounts
• Track account performance & competitor activities
Locations: All major cities across India (East, West, North, South zones)
Experience: Minimum 3 years of work experience in IV Fluids / Critical Care sales
Key Responsibilities:
• Promote IV fluids portfolio to doctors, pharmacists and hospitals
• Generate prescriptions and ensure regular follow-up with medical professionals
• Achieve assigned monthly sales targets in territory
• Execute marketing strategies, product detailing & CME activities
• Expand presence in hospitals, institutions & retail chemists
Qualifications:
• Graduate in Science / Pharmacy preferred (any graduate with strong pharma sales exposure may apply)
• Strong communication, relationship management & product knowledge
Location: Silica Plant
Experience: 7–12 years (Pharma / Healthcare industry preferred) with strong ERP & MIS implementation background
Key Responsibilities:
• Lead ERP system implementation, customization and integration across sales, supply chain, billing and field force modules.
• Ensure smooth functioning of MIS (Management Information System) for accurate and timely reporting.
• Develop dashboards, sales analysis, inventory reports and business intelligence tools for management decision-making.
• Coordinate with different departments (Sales, Marketing, Finance, Supply Chain, HR) to ensure process automation through ERP.
• Train and support employees in ERP usage and resolve operational issues.
• Maintain data security, compliance and integrity of ERP & MIS systems.
• Evaluate and implement new technologies to improve efficiency.
• Liaise with IT vendors, ERP consultants and management for continuous system upgrades.
Skills & Competencies:
• Strong knowledge of ERP platforms (SAP / Oracle / Microsoft Dynamics / Tally ERP or Pharma ERP systems).
• Expertise in MIS reporting, dashboards and advanced Excel / Power BI tools.
• Experience in pharma/healthcare sales & supply chain reporting preferred.
• Strong analytical, problem-solving and cross-functional coordination skills.
• Ability to manage ERP projects end-to-end (planning to execution).
Qualifications:
• Graduate/Post-Graduate in IT / Computer Science / Business Analytics / Management
• Certification in ERP platforms (SAP, Oracle, etc.) will be an added advantage.
⸻
✅ This role is critical for ensuring data-driven decision-making and digital transformation at Silica Healthcare.
Location: Silica Healthcare – Plant
Silica Healthcare Pvt. Ltd. is looking for a highly skilled and experienced professional to join our team as a Distribution & MIS Manager.
Key Responsibilities:
• Manage end-to-end distribution operations to ensure timely and accurate supply of products.
• Oversee and monitor MIS reporting systems (SAP & ERP) for effective decision-making.
• Develop and streamline processes to optimize inventory management, dispatch and logistics.
• Coordinate with internal departments and external partners for smooth operations.
• Ensure compliance with company standards and regulatory requirements.
Requirements:
• Minimum 6 years of experience in Distribution & MIS in the pharmaceutical/healthcare/related industry.
• Strong working knowledge of SAP & ERP systems.
• Excellent leadership, coordination and problem-solving skills.
• Ability to work in a fast-paced, plant-based environment.
Location: Silica Healthcare Pvt. Ltd.
Silica Healthcare Pvt. Ltd. is inviting applications for the position of Product Manager. We are looking for a highly motivated and experienced professional to lead our product strategy and drive market growth.
Key Responsibilities:
• Manage the entire product lifecycle – from development to launch and beyond.
• Conduct market research, competitor analysis and sales forecasting.
• Develop and implement branding and promotional strategies.
• Collaborate with R&D, manufacturing, sales and marketing teams for effective execution.
• Provide scientific training and product knowledge support to the sales team.
• Track product performance and take corrective actions to achieve business goals.
Requirements:
• Minimum 6 years of experience as a Product Manager in the pharmaceutical/healthcare industry.
• Strong knowledge of therapeutic areas, branding and market dynamics.
• Proficiency in MIS tools, data analysis and communication skills.
• Ability to work cross-functionally with strong leadership qualities.
Location: Silica Healthcare – Plant
Silica Healthcare Pvt. Ltd. is looking for a highly skilled and experienced professional to join our team as a Distribution & MIS Manager.
Key Responsibilities:
• Manage end-to-end distribution operations to ensure timely and accurate supply of products.
• Oversee and monitor MIS reporting systems (SAP & ERP) for effective decision-making.
• Develop and streamline processes to optimize inventory management, dispatch and logistics.
• Coordinate with internal departments and external partners for smooth operations.
• Ensure compliance with company standards and regulatory requirements.
Requirements:
• Minimum 6 years of experience in Distribution & MIS in the pharmaceutical/healthcare/related industry.
• Strong working knowledge of SAP & ERP systems.
• Excellent leadership, coordination and problem-solving skills.
• Ability to work in a fast-paced, plant-based environment.
Location: Silica Healthcare Pvt. Ltd. – Plant
Silica Healthcare Pvt. Ltd. is seeking an experienced and dynamic professional to lead our Distribution & Logistics operations. The ideal candidate will be responsible for ensuring seamless supply chain efficiency, timely product delivery and compliance with company standards.
Key Responsibilities:
• Manage and oversee end-to-end distribution & logistics operations.
• Ensure timely dispatches of products to distributors, stockists and customers.
• Optimize transportation, warehousing and inventory management.
• Implement and monitor MIS (SAP/ERP) for accurate reporting and tracking.
• Coordinate with sales, production and QA/QC teams for smooth supply chain operations.
• Ensure compliance with GMP, GDP and regulatory guidelines in distribution.
• Drive cost efficiency and process improvement initiatives.
Requirements:
• Minimum 6–8 years of experience in Distribution & Logistics in the pharma/healthcare industry.
• Strong knowledge of SAP/ERP, MIS systems and supply chain practices.
• Excellent leadership, planning and coordination skills.
• Proven ability to manage teams and vendor relationships.
• Must be willing to work at plant location.
Location: Silica Healthcare Pvt. Ltd. – Corporate/Plant
Silica Healthcare Pvt. Ltd. is looking for an experienced and proactive Export & Liaisoning Manager to handle international trade operations and regulatory coordination. The role demands strong expertise in export documentation, regulatory compliance and liaisoning with authorities.
Key Responsibilities:
• Manage end-to-end export operations including documentation, shipping and regulatory requirements.
• Liaise with DGFT, Customs, Excise, FDA and other regulatory bodies for approvals and compliance.
• Ensure timely order processing, dispatches and export logistics.
• Maintain and monitor export licenses, permissions and statutory records.
• Coordinate with internal teams (Production, QA/QC, Distribution) to meet international client requirements.
• Handle liaisoning activities with government departments and industry associations.
• Explore and develop new international markets and support business expansion.
Requirements:
• Minimum 6–8 years of experience in Exports & Regulatory Liaisoning in the pharma/healthcare industry.
• Strong knowledge of export-import policies, DGFT norms, customs clearance and regulatory documentation.
• Familiarity with WHO/GMP/USFDA/ROW markets compliance preferred.
• Excellent communication, negotiation and coordination skills.
• Must have hands-on experience in SAP/ERP systems for export documentation
Key Responsibilities:
• Lead the Quality Control department ensuring compliance with GMP and regulatory guidelines.
• Oversee raw material, in-process and finished product testing.
• Ensure timely calibration and validation of instruments.
• Manage documentation, reports and analytical method development.
• Lead, mentor and train QC team members.
Requirements:
• Minimum 8–10 years of experience in pharmaceutical QC.
• Strong expertise in analytical techniques, instrumentation and regulatory standards.
• Proven leadership and problem-solving skills.
Key Responsibilities:
• Lead the Quality Assurance department, ensuring compliance with cGMP and regulatory requirements.
• Oversee documentation, audits and quality management systems (QMS).
• Ensure batch record reviews, CAPA implementation and deviation handling.
• Manage regulatory inspections, internal audits and customer audits.
• Drive continuous improvement in quality systems and processes.
Requirements:
• Minimum 8–10 years of experience in pharmaceutical QA.
• Strong knowledge of GMP, QMS and regulatory audits (USFDA/WHO/EU GMP).
• Excellent leadership, communication and compliance management skills.
Location: Silica Healthcare Pvt. Ltd. – Plant
Silica Healthcare Pvt. Ltd. is looking for a highly skilled professional to manage and oversee Production & Packaging operations at our manufacturing facility. The role requires strong leadership and technical expertise to ensure compliance, efficiency and quality in every batch.
Key Responsibilities:
• Plan, organize and supervise production and packaging activities as per GMP guidelines.
• Ensure timely execution of manufacturing schedules to meet market demand.
• Oversee packaging operations including line clearance, labeling and batch reconciliation.
• Monitor compliance with SOPs, cGMP and regulatory standards.
• Coordinate with QA/QC, engineering and supply chain teams for smooth operations.
• Implement continuous improvement initiatives for productivity, cost optimization and waste reduction.
• Train and develop the production & packaging team to ensure high performance.
Requirements:
• Minimum 6–10 years of experience in pharmaceutical production & packaging.
• Strong knowledge of GMP, GDP and regulatory compliance.
• Hands-on experience with manufacturing & packaging equipment.
• Good knowledge of documentation, batch records and validation processes.
• Leadership, planning and problem-solving skills essential.
Company: Silica Healthcare (HQ)
Location: Delhi
Qualification & Experience:
• Must be Bachelor in Medicine (MBBS or equivalent recognized degree)
• Strong exposure to Intravenous (IV) and Intramuscular (IM) fluids segment
• 6–10 years of relevant experience in Medical Affairs & Pharma Marketing
• Proven ability to bridge clinical knowledge with marketing strategy
• Strong leadership, analytical and communication skills
Key Responsibilities:
• Lead the medico-marketing strategy for IV & IM fluid products
• Develop and execute scientific marketing campaigns aligned with brand objectives
• Provide medical insights to marketing, sales and regulatory teams
• Conduct training sessions for field force and distributors
• Build strong collaborations with Key Opinion Leaders (KOLs), doctors and healthcare professionals
• Support regulatory submissions and ensure compliance with NPPA/DPCO and medical ethics
• Review, validate and approve medical content, product monographs and promotional materials
• Identify new molecules and opportunities in IV/IM fluids to expand Silica Healthcare’s portfolio
Why Join Us?
• Be a part of Silica Healthcare HQ, Delhi driving growth in the complete IV & IM solutions segment
• Leadership role with strategic impact and innovation opportunities
• Competitive salary, incentives and career growth
